Hydroxyurea: an ally in the treatment of sickle cell disease.
December 6, 2021
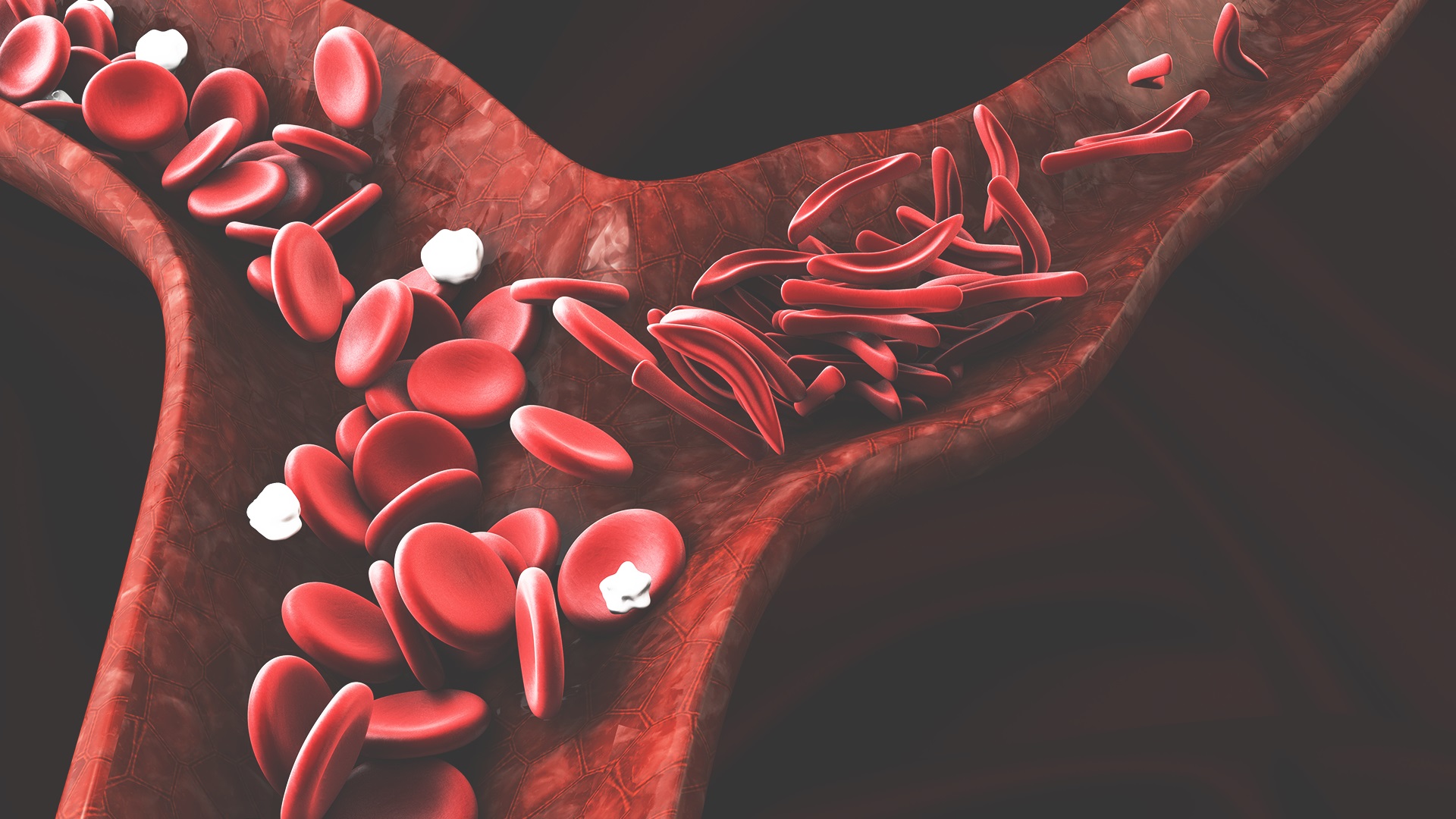
Sickle cell disease is a genetic disease caused by changes in red blood cells, which predominantly affects black people. The disease is characterized by hemolytic anemia, vaso-occlusive crises, relentless end-organ damage, and premature death.
Red blood cell transfusion and the use of hydroxyurea are the main disease-modifying therapies. Gene therapy and hematopoietic stem cell transplantation are also used as curatives, although the difficulties for transplantation are great, including lack of suitable donors, rejection, long-term adverse effects, prognostic uncertainty, and poor organ function. target.1
Hydroxyurea is the most widely used drug worldwide and one of the safest for the treatment of severe cases of sickle cell disease.2 The drug has been used since the 1980s. The FDA – Food and Drug Administration, the American body that approves the use of drugs in that country, approved hydroxyurea for the treatment of adults with sickle cell disease in 1998. In 2017, it also approved its use to treat children with the disease.3
How hydroxyurea works and helps in the treatment of sickle cell disease.
Hydroxyurea helps red blood cells remain rounder and more flexible, and therefore less likely to become sickle-shaped (hence the disease's name). Hydroxyurea does this by increasing hemoglobin F – the fetal hemoglobin that exists in all newborns. With high levels of hemoglobin F the erythrocytes are less susceptible to problems. By preventing the deformation of blood cells, hydroxyurea reduces the problems that sickle cell disease causes, such as pain crises, episodes of acute chest syndrome, the need for blood transfusions and hospital admissions.2
New treatment for sickle cell disease approved by Anvisa, for use in patients from 2 years of age.
Hydroxyurea represents a better quality of life for people with sickle cell disease. That is why the approval, in December 2021, by Anvisa, of the first hydroxyurea-based treatment with indication on the leaflet and precise and safe dosage for patients from 2 years of age, is an achievement and a hope for the Brazilian population of black ethnicity, the most benefited by the new treatment. The drug, approved and already in use in Europe and the United States, will soon be available throughout Brazil.
Masters is always at the side of patients, doctors, hospitals, public agencies and health plans in the search and availability of new and better health treatments around the world.
#hydroxyurea #sickle cell disease 1TP3Sickle cell tanemia #hereditary diseases #genetic diseases #gene therapy 1TP3Blackmoth #black population #public health #emedule transplant #stem cell #fda #foodanddrugadministration
#masters #masterspharma #mastersspecialitypharma
References:
- May Clinic. Advances in the Treatment of Sickle Cell Disease. Available in: https://www.mayoclinicproceedings.org/article/S0025-6196(18)30585-8/fulltext Accessed in December 2021.
- Sickle cell disease and the use of hydroxyurea. Available in: https://portal.fiocruz.br/video/doenca-falciforme-e-o-uso-da-hidroxiureia Accessed in December 2021.
- American Society of Hematology. Hydroxyurea for Sickle Cell Disease. Available in: https://www.hematology.org/-/media/Hematology/Files/Education/Hydroxyurea-Booklet.pdf Accessed in December 2021.




